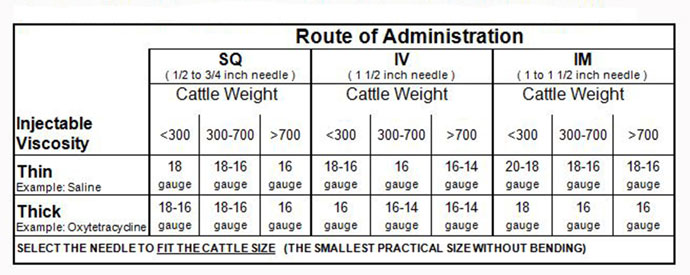
This chart shows recommended needle size, based on animal size, injection site and viscosity of the product. (Source: National Beef Quality Assurance Training Manual)
Nebraska BQA: Antibiotic Use Guidelines
By Rob Eirich
Nebraska Beef Quality Assurance
University of Nebraska-Lincoln Extension
Selection of an animal health product is the first step in antibiotic use. The USDA Food and Drug Administration requires that all animal health products be labeled for proper usage. When selecting a product, veterinarians and producers must consider cause for treatment, dosage, method of administration, and withdrawal time.
The FDA requires that the label be followed and all extra-label drug use must have a veterinarian prescription. BQA also recommends using antibiotics that are administered subcutaneously (SQ – under the skin), intranasal (IN), oral, or intravenous (IV – in the vein). Only use intramuscular (IM – in the muscle) injections when required by the product label. A strong veterinarian-client-patient relationship is highly valuable when selecting products.When administering product, use clean and sterile syringes and needles. Use a proper-size needle based on animal size, route of administration, and viscosity of product. The most common needle sizes are 16 or 18 gauge, length ~½ inch to 1 inch. Needles should be replaced if bent, damaged, or dull and after every 10-15 injections. The accompanying table can assist in needle selection.
All injections should be administered in the triangle area located in the neck region. If administering intramuscularly (IM), only 10 cubic centimeters (cc) per injection site is recommended, as well as multiple sites two to four inches apart. Using proper handling and restraint methods will assist in administering animal health products correctly.Producers must always maintain animal health records. These records should contain animal identification, diagnosis, treatment date, product used, location and route of administration, dosage, and withdrawal time and date. They should be kept on file for a minimum of three years.
Cattlemen strive to prevent animal diseases through strong Herd Health and Vaccination Protocols, but when diagnosis requires treatment they are committed to following proper antibiotic usage.